A collaborative team of scientists from the Netherlands and Australia has made a significant breakthrough in the field of chemical systems by developing a novel enzyme network that can adapt to its environment. This network, which features competing peptides and proteases, demonstrates the capability to respond to various external stimuli, effectively mimicking decision-making processes once thought to be exclusive to complex organisms or advanced computing systems.
Advancing Chemical Decision-Making
Traditionally, the ability to interpret and respond to environmental changes has been reserved for living organisms and sophisticated computers. These systems rely on their ability to process signals and act accordingly based on predefined instructions. However, the pioneering work of these researchers marks a turning point in chemical engineering, where a simple mixture of peptides and enzymes can exhibit complex behavior.
The research, published in Nature Chemistry on November 12, 2025, outlines how this innovative chemical network can classify both chemical and physical signals. Notably, it can accurately sense temperature fluctuations within the range of 25–55°C with a precision of about 1.3°C. This capability allows the enzyme network to perform tasks akin to those of neural systems, such as making decisions and adjusting its responses based on environmental inputs.
Emulating Biological Complexity
Living cells continuously gather information from their surroundings, detecting variables like nutrients, temperature, and light. Researchers have previously explored the use of network motifs—patterns found in complex biological systems—to design synthetic reaction networks that mimic these processes. While earlier attempts fell short of capturing the full complexity of living organisms, the latest research leverages recursive interactions to achieve a higher level of sophistication.
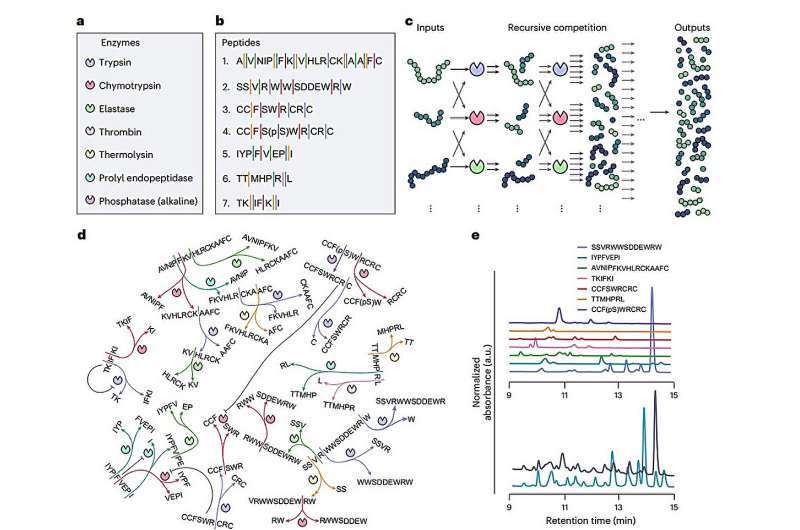
The team constructed a recursive enzymatic competition network (ERN) that incorporates seven enzymes and seven peptides, creating a dynamic environment for molecular computing. As these peptides compete for access to the enzymes, they undergo repeated cleavage, resulting in a constantly changing mixture of chemical fragments. This ongoing reaction generates a nonlinear network of enzymatic processes, allowing for a versatile response to varying initial inputs, including changes in peptide concentration or alterations in the physical environment.
Real-time measurements of these chemical fragments are facilitated by a mass spectrometer. The resulting data is analyzed using a simple algorithm known as a linear readout layer. This system decodes fragment patterns to produce final decisions or predictions, enabling tasks such as temperature sensing and detecting periodic changes in light or time.
The implications of this research are profound. The capabilities demonstrated by the ERN suggest potential applications in developing smarter, more adaptive biosensors and materials. Such innovations could significantly impact fields ranging from healthcare to advanced technology, providing tools that better respond to their environments.
This remarkable advancement represents a crucial step towards creating chemical systems that can operate with the complexity and adaptability characteristic of biological life. The ongoing research promises to enhance our understanding of molecular interaction and decision-making processes, potentially leading to a new era in chemical and materials science.