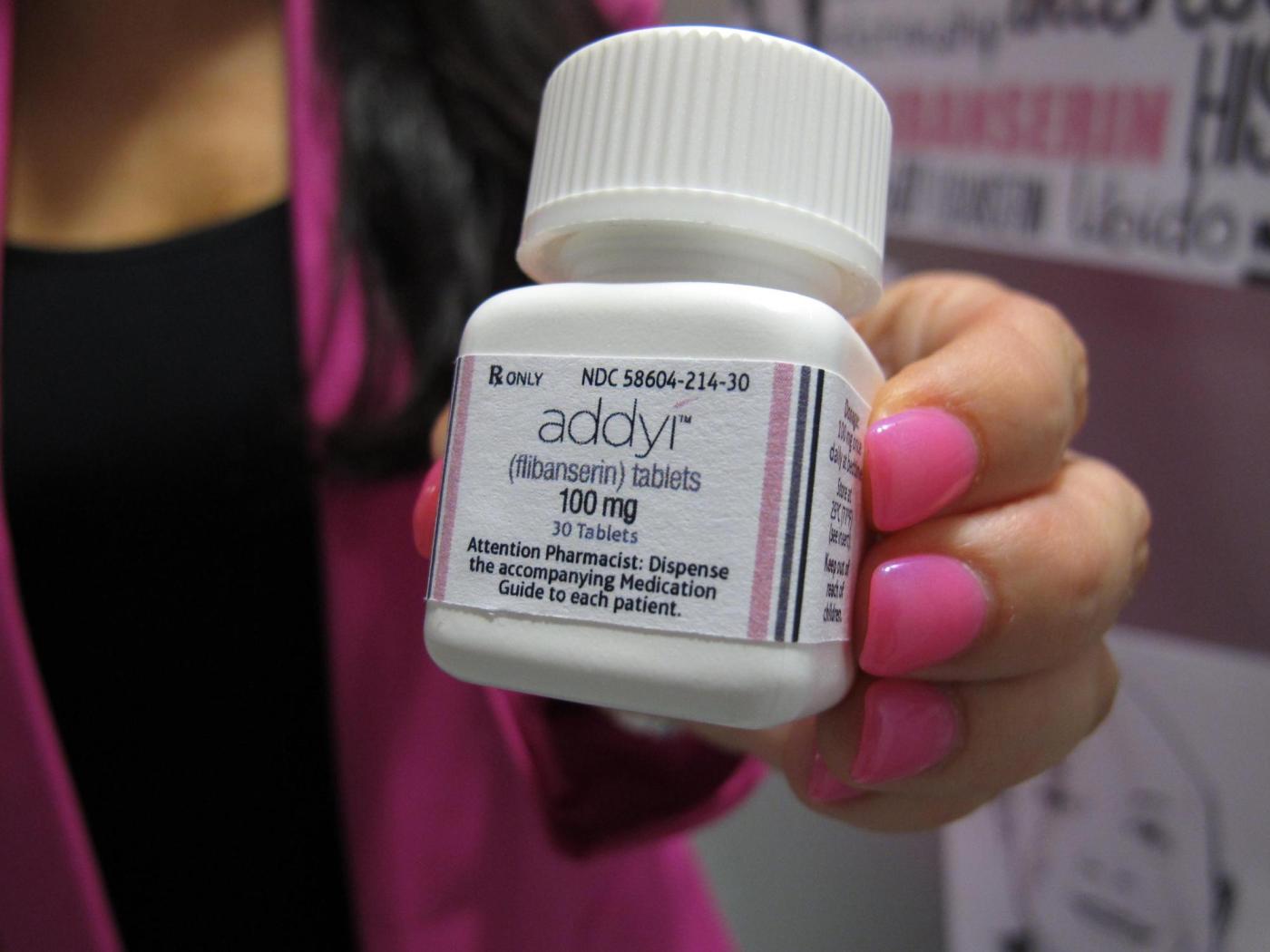
U.S. health officials have expanded the approval of the libido-enhancing medication, Addyi, to include women over the age of 65 who have gone through menopause. The Food and Drug Administration (FDA) made this announcement on March 4, 2024, marking a significant change in the drug’s usage profile since its initial approval a decade ago.
Originally approved in 2015 for premenopausal women suffering from low sexual desire related to emotional stress, Addyi has now been authorized for a broader demographic. This shift aims to address the medical condition known as hypoactive sexual desire disorder, which affects a considerable number of women, particularly those experiencing hormonal changes post-menopause.
Challenges and Controversies Surrounding Addyi
Manufactured by Sprout Pharmaceuticals, Addyi was initially anticipated to become a leading treatment option for women’s sexual health. However, the drug has faced challenges due to side effects, including dizziness and nausea. Notably, it carries a boxed warning from the FDA, indicating serious risks associated with alcohol consumption, which can lead to dangerously low blood pressure and fainting.
Sales of Addyi have not met initial expectations, prompting concerns about its effectiveness and safety. In response to these challenges, the FDA approved a second treatment for low female libido in 2019, an on-demand injection that operates through different neurological pathways.
Cindy Eckert, CEO of Sprout Pharmaceuticals, emphasized the significance of this new approval, stating it reflects a decade of persistent efforts to reshape the understanding and prioritization of women’s sexual health. The company made the announcement through a press release, highlighting its commitment to addressing this often-overlooked aspect of women’s health.
Understanding the Condition and Its Impact
The condition of hypoactive sexual desire disorder has been recognized since the 1990s and is thought to affect a substantial portion of American women. Following the success of Viagra for men, pharmaceutical companies began investing heavily in research focused on female sexual dysfunction. However, diagnosing this condition poses challenges due to the numerous factors that can influence libido, particularly after menopause when hormonal fluctuations can lead to various biological changes.
Healthcare professionals are advised to consider a range of issues, including relationship dynamics, medical conditions, and mental health factors, before prescribing medications like Addyi. There remains a lack of consensus among psychologists regarding whether low sexual desire should be classified as a medical issue.
The journey of Addyi to approval was not straightforward. The FDA initially rejected the drug twice due to concerns over its modest effectiveness and potential side effects. Its eventual approval was significantly influenced by advocacy efforts from the company and supporters who framed the lack of treatment options for women as a pressing women’s rights issue.
The FDA’s recent decision to broaden the use of Addyi represents a notable development in the landscape of women’s health, acknowledging the complexities surrounding libido and aiming to provide more options for those affected by low sexual desire.
This update underscores the ongoing dialogue surrounding women’s sexual health and the importance of recognizing and addressing their unique medical needs.