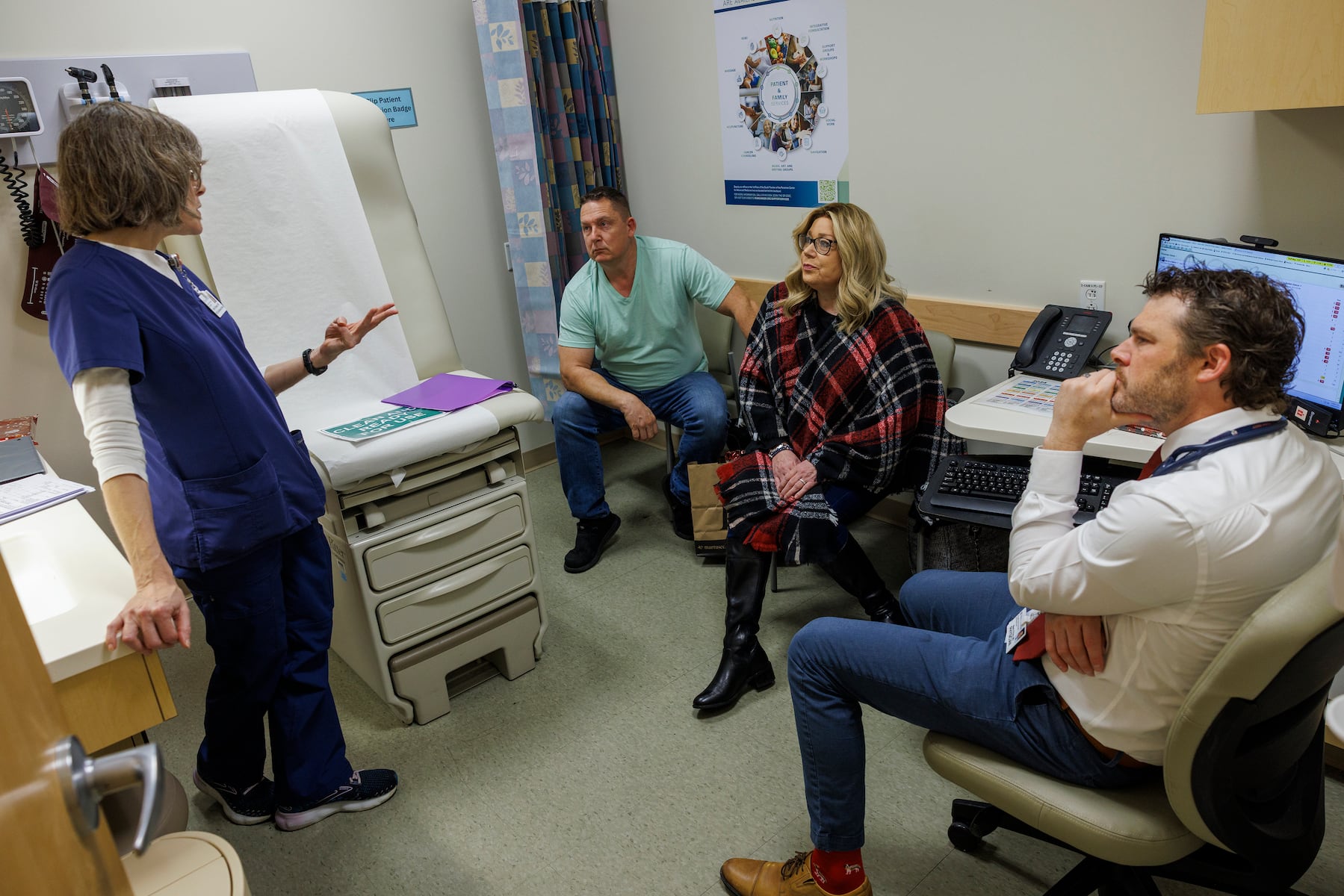
In a significant development for pancreatic cancer treatment, a new drug known as daraxonrasib is showing promising results in clinical trials at Penn Medicine’s Abramson Cancer Center. The drug, a type of KRAS inhibitor, offers hope to patients like Irene Blair, a 59-year-old grandmother from Newark, Delaware, who was diagnosed with stage 4 pancreatic cancer. After being given only six to eight months to live, Blair entered a phase 3 trial in July 2023, leading to remarkable improvements in her condition.
Pancreatic cancer is notorious for its high mortality rate, with only 13% of patients surviving five years post-diagnosis, making it one of the deadliest forms of cancer. The advent of KRAS inhibitors like daraxonrasib marks a potential breakthrough, as they target a protein that drives the growth of this aggressive cancer. While daraxonrasib is not a cure, early clinical trials reveal it may significantly extend survival for patients, doubling the average survival time from chemotherapy from approximately 7 months to 15.6 months.
The urgency surrounding daraxonrasib has increased, especially after former Nebraska Senator Ben Sasse recently disclosed his diagnosis of stage-four pancreatic cancer, stating, “I’m gonna die.” The U.S. government has expedited the review process for this promising drug, reflecting its potential impact on patient survival.
Blair’s oncologist, Mark O’Hara, who leads several KRAS inhibitor trials at Penn, expressed optimism about the drug’s effectiveness. “In pancreatic cancer, for too long, we haven’t had effective therapies beyond just chemotherapy,” he noted. Following her treatment with daraxonrasib, Blair reported a significant reduction in cancer-associated pain within three weeks. Scans in October showed her tumors were either stable or decreasing, and her most recent scan in December confirmed that her cancer had not progressed.
“This is a huge improvement compared to the debilitating side effects of chemotherapy,” Blair shared, recalling her previous struggle with weight loss and fatigue. Now, she is eager to enjoy life, having retired from her real estate job in May. Plans for family visits to California and Florida fill her with hope, as she contemplates what the future holds.
The journey to develop effective KRAS inhibitors has been long, with research dating back to 1982. The KRAS protein functions as a “gas pedal” for cancer growth when mutated, a condition present in approximately 25% of all cancers, particularly aggressive forms found in the pancreas, lungs, and colon. The first KRAS inhibitors received FDA approval in 2021 for lung cancer, and now, drugs like daraxonrasib are positioned as potential game-changers for pancreatic cancer patients.
In clinical trials, over 90% of participants in a phase 1 study experienced stabilization of their pancreatic cancer, while about 30% saw notable tumor shrinkage. The oral medication consists of three daily pills, with manageable side effects primarily including facial rashes, diarrhea, nausea, and mouth sores. O’Hara emphasized that these symptoms are far less debilitating than those associated with chemotherapy.
As research continues, the focus remains on how long patients can benefit from these therapies. With the prospect of improved quality of life and extended survival, patients like Blair are hopeful for what lies ahead. “I want to be able to give KRAS inhibitors to all my patients right now,” O’Hara concluded, reflecting the urgent need for effective treatments in the fight against pancreatic cancer.