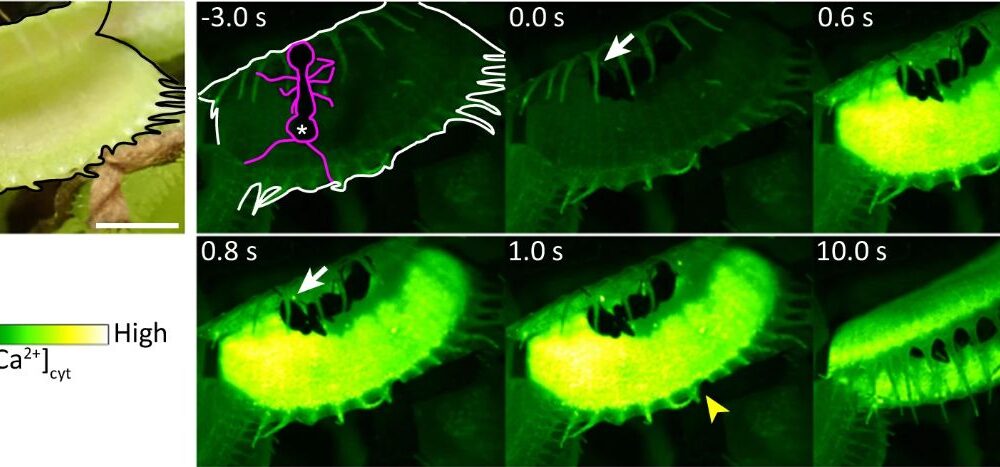
The Venus flytrap, known scientifically as Dionaea muscipula, has long intrigued scientists due to its unique ability to swiftly capture prey. Recent research led by Hiraku Suda and his team has uncovered the role of a specific mechanosensor, termed DmMSL10, which is crucial for the plant’s rapid response to stimuli. This study, published in Nature Communications, provides new insights into the mechanisms that enable these carnivorous plants to respond so quickly.
The Venus flytrap uses specialized sensory hairs on its leaves that react to movement. These hairs are sensitive to mechanical stimuli and trigger a release of calcium ions, prompting the trap to close around unsuspecting insects. While the general function of these hairs was known, the precise mechanisms remained unclear until now. The team’s research indicates that the DmMSL10 mechanosensor is fundamental in detecting small movements, which is essential for effective prey capture.
In their experiments, the researchers created a knockout variant of the DmMSL10 gene, effectively disabling the mechanosensor. They discovered that while both the wild-type and knockout plants could respond to mechanical stimulation, the rate of action potential generation was markedly lower in the knockout variant. Specifically, the wild-type plants continued to generate action potentials even after the stimulation had stopped, indicating a robust processing capability for detecting prey.
To further illustrate the findings, the team conducted an experiment where ants were allowed to roam on the leaves of both the wild-type and knockout plants. The wild-type Venus flytrap successfully captured the first ant that crossed its path, while the knockout variant failed to react, even after multiple ants walked on its leaves. This stark difference highlights the critical role of DmMSL10 in the plant’s ability to respond to potential food.
Understanding the function of DmMSL10 not only sheds light on the biology of the Venus flytrap but also opens avenues for exploring similar mechanosensory mechanisms in other organisms, including animals. The study enriches our comprehension of plant responses and their evolutionary adaptations, suggesting that the ability to develop such sensory systems may have evolved in parallel across different life forms.
As research continues, the findings could lead to broader implications for understanding how plants interact with their environment and could inspire advancements in bioengineering and robotics, where similar sensory systems might be mimicked for various applications. The exploration of such natural mechanisms reminds us of the complexity and wonder of the biological world.